
Moderna sues Pfizer / BioNTech for COVID vaccine patent infringement
On August 26, 2022, biotechnology company Moderna filed lawsuits against pharmaceutical giant Pfizer and its German partner BioNTech for the alleged infringement of patented mRNA technology in the development of the "Comirnaty" COVID-19 vaccine.
The lawsuits, deposited in the U.S. District Court for the District of Massachusetts (United States) and the Regional Court of Düsseldorf (Germany), claim that the creators of the joint Pfizer–BioNTech vaccine made unauthorized use of patents filed by Moderna over the period 2010-2016.
"We are filing these lawsuits to protect the innovative mRNA technology platform that we pioneered, invested billions of dollars in creating, and patented during the decade preceding the COVID-19 pandemic," stated Moderna's Chief Executive Officer Stéphane Bancel in a press release.
Though Intellectual Property (IP) disputes are not uncommon in the pharmaceutical industry, the scale and prominence of this litigation are of rare magnitude.
What is mRNA technology?
At the heart of the IP dispute are immunological techniques that utilize mRNA – short for "messenger ribonucleic acid." Unlike DNA, which has a familiar double-helical arrangement, RNA encodes genetic information in a single, looped strand and is the primary structure in virus genomes. As its name implies, mRNA is used in more complex cells to relay genetic information, specifically, during protein synthesis. In essence, the mRNA molecule acts as a short-lived "burn after reading" data packet that is first transcribed from DNA, transferred within the cell, read, translated to produce the corresponding protein and then degraded.
Synthetic-mRNA vaccines exploit this mechanism to cause the patient's body to produce viral spike proteins. Despite being neither a virus nor dangerous, these identifying spike proteins – called antigens – trigger the body's normal immune response and are destroyed. Subsequently, the immune system retains a memory of the encountered antigen and is able to recognize and respond to it more quickly in the event of an actual viral infection.
Not all vaccines for COVID-19 rely on mRNA processes. For instance, the Oxford–AstraZeneca COVID‑19 vaccine uses the older and more pervasive method of delivering antigens by way of a modified virus. In this approach, a weakened or "dead" virus is injected into the patient in order to stimulate an immune response – the antigens being present in the dose. In an mRNA vaccination, the body is fooled into producing the required antigens itself by artificial "blueprints." Both methods have been proven to be highly effective.
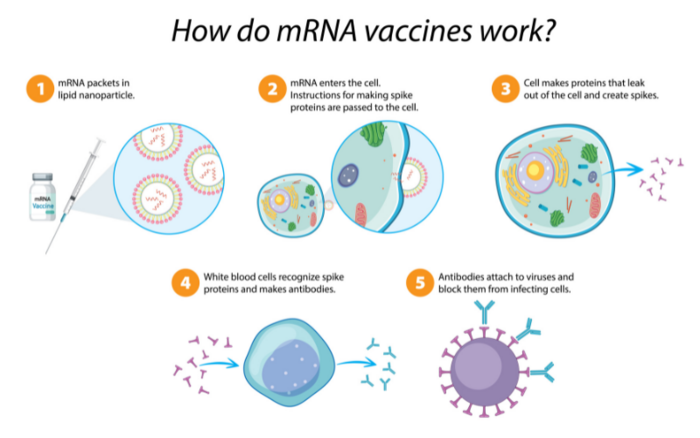
Prior to the expedited approval of the first COVID-19 vaccines by national regulators in late 2020, no pharmaceutical product based on mRNA technology had seen public deployment. Curiously, the Pfizer-BioNTech and Moderna vaccines were granted emergency authorization by the U.S. Food and Drug Agency just one week of each other in December 2020.
Why now?
In October 2020, Moderna announced a voluntary moratorium on enforcing its patent rights pertaining to COVID-19 vaccines globally. This was done to encourage the creation and distribution of vital medications at a time of global emergency. Later, in light of the reduced severity of the pandemic and the widespread availability of various vaccines to high-income nations, Moderna reversed this policy in those less-deprived countries. From March 2022, all IP rights were declared fully enforceable except in 92 low- and middle-income countries, referred to as advance market commitment (AMC) territories.
Moderna Chief Legal Officer Shannon Thyme Klinger: "Outside of AMC 92 countries, where vaccine supply is no longer a barrier to access, Moderna expects Pfizer and BioNTech to compensate Moderna for Comirnaty's ongoing use of Moderna's patented technologies."
As such, the unspecified damages sought from Pfizer and BioNTech are to be calculated only from March of this year. Nevertheless, these figures would still be substantial, considering Pfizer projected its 2022 revenue to be approximately $32 billion USD from Comirnaty alone. Similarly, relative newcomer BioNTech saw its annual profits skyrocket from €15.2 million in 2020 to €10.3 billion one year later.
In its own statement, BioNTech upheld the independence and uniqueness of its research and of the Comirnaty vaccine. "BioNTech's work is original, and we will vigorously defend against all allegations of patent infringement. BioNTech also values and respects valid and enforceable intellectual property rights of others and remains confident in its intellectual property." The firm went on to express dismay at Moderna's action: "It is an unfortunate but rather regular occurrence that other companies make allegations that a successful product potentially infringes their intellectual property rights."
Both BioNTech and Pfizer have declined to comment on their legal strategies.
Patents in medicine
The purpose of the patent regimes in pharmaceutical research is to secure sufficient compensation to recoup extremely high development costs and fund additional projects. As such, Moderna's time- and country-specific waiver of IP rights is just one possible approach to the dilemma of ensuring adequate drug accessibility in a crisis. However, the role of exclusivity rights in the fight against counterfeit medications should not be overlooked.
Dennemeyer's legal experts in the United States, Germany and worldwide will follow these cases closely and continue to provide their professional insight as the matter progresses.
Filed in

Explore the latest IP dramas, from an Olympic trademark dispute to challenges in drug patenting and a lawsuit for allowing music piracy.



